The Science of Aging: An IOA Newsletter, Summer 2025 Edition | DIGITAL VERSION 
A LOOK INSIDE:
- FDA Approves First Blood Test Used in Diagnosing Alzheimer's Disease
- The Cost of Dementia
- Awards & Accolades
- IOA-Funded Research: Newly funded Alzheimer's Disease Therapeutics Accelerator Project
-
Farraday Johnson: From STAR Intern to Penn Memory Center Coordinator
FDA Approves First Blood Test Used in Diagnosing Alzheimer's Disease
The U.S. Food and Drug Administration (FDA) has officially cleared the first-ever blood test to aid in the diagnosis of Alzheimer’s disease — a milestone many experts say could transform how the disease is detected and managed. The newly approved test, The Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio, measures specific proteins in the blood that are associated with amyloid plaques in the brain.
Until now, diagnosing Alzheimer’s has often relied on more invasive, expensive procedures like PET scans or spinal taps. This blood test offers a much simpler, less invasive option, with the potential of finally making earlier and more accessible diagnosis a reality.
“The advancement of blood-based biomarkers for Alzheimer’s Disease has been truly breathtaking over the last few years,” said David A. Wolk, MD, Co-Director of the Institute on Aging and Penn Memory Center. “FDA approval of this test is a landmark for the field and likely the beginning of an era in which blood tests will play a critical role in our care of patients.”
The FDA’s clearance of the test marks a major step forward in the ongoing fight against Alzheimer’s and reflects growing momentum in the development of accessible, reliable diagnostic tools. Experts are hopeful that this will not only improve diagnosis but also accelerate clinical trials and, ultimately, lead to better treatments.
Read the full FDA Press release here.
Source: U.S. Food and Drug Administration, FDA Clears First Blood Test to Aid in the Diagnosis of Alzheimer’s Disease, June 2024.
A newly developed model from the University of Southern California (USC) Schaeffer Center is shedding light on the enormous and growing financial impact of Alzheimer’s disease and related dementias in the United States. The U.S. Dementia Cost Model projects that the national costs of dementia care will soar to more than $780 billion this year.
Among the experts leading this collaborative effort — which includes members from the Alzheimer’s Association and the University of Pennsylvania — are Norma Coe, PhD, Professor of Medical Ethics and Health Policy at the Perelman School of Medicine and leader of the IOA’s Division of Epidemiology, Social Science, and Policy, and Eric Roberts, PhD, Associate Professor of General Internal Medicine at the Perelman School of Medicine and Senior Fellow at Penn’s Leonard Davis Institute of Health Economics.
The tool provides detailed estimates of dementia’s economic impact, considering not just healthcare costs, but also the often-overlooked expenses related to caregiving, long-term services, and support for individuals living with cognitive decline. It offers policymakers, healthcare providers, and families new insight into where costs are rising—and how better care planning and policy could help address the challenge.
With dementia cases expected to grow substantially in the coming years, the model represents an important step toward understanding and preparing for the personal and economic realities of this complex disease.
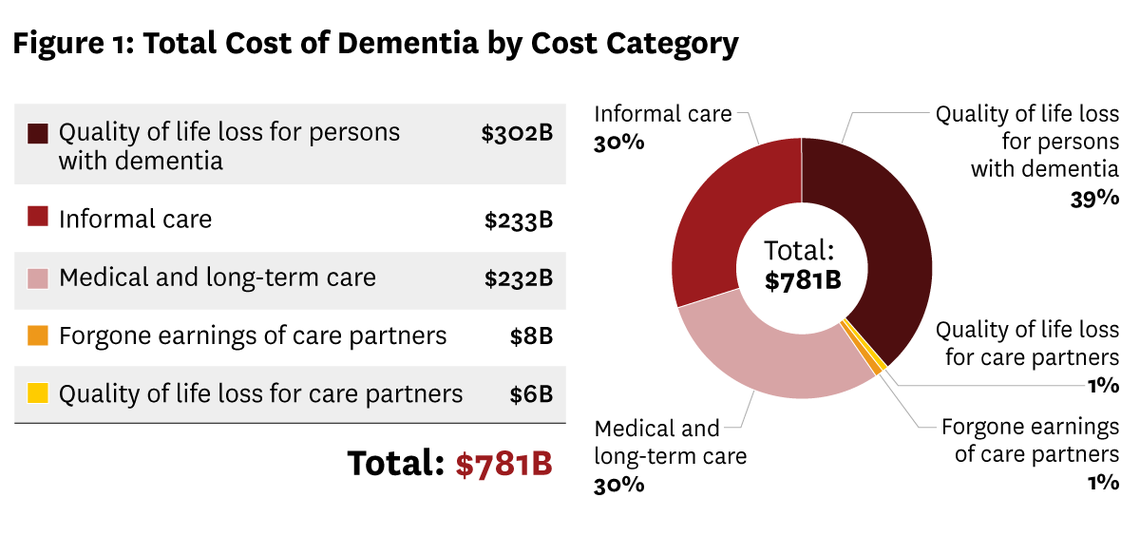
Source: USC Schaeffer Center for Health Policy & Economics, United States Cost of Dementia Model, April 2025.
 Edward B. Lee, MD, PhD, Named President of the American Association of Neuropathologists
Edward B. Lee, MD, PhD, Named President of the American Association of Neuropathologists
Edward (Eddie) B. Lee, MD, PhD, Associate Professor of Pathology & Laboratory Medicine at the University of Pennsylvania and Co-Director of the IOA, has been named President of the American Association of Neuropathologists for 2024–2025. In this role, Dr. Lee will help guide the association’s efforts to advance research, education, and collaboration in the field of neuropathology.
 Virginia M.Y. Lee, PhD, Elected to National Academy of Sciences
Virginia M.Y. Lee, PhD, Elected to National Academy of Sciences
Virginia M.Y. Lee, PhD, Director of Penn’s Center for Neurodegenerative Disease Research (CNDR), has been elected to the National Academy of Sciences, one of the highest honors in science. She is recognized for her groundbreaking research in neurodegenerative diseases.
 John Detre, MD, Receives International Society for Magnetic Resonance Medicine’s Gold Medal Award
John Detre, MD, Receives International Society for Magnetic Resonance Medicine’s Gold Medal Award
John Detre, MD, Professor of Neurology and Radiology at the Perelman School of Medicine, has received the 2025 Gold Medal Award from the International Society for Magnetic Resonance Medicine. According to the ISMRM, this award recognizes Dr. Detre’s “pioneering and enduring contributions to the development of arterial spin labeled (ASL) perfusion MRI and its applications in basic and clinical neuroscience.”
Principal Investigators: Ian A. Blair, George Kannarkat, David J. Irwin, Katheryn A.Q. Cousins
Funded by the IOA’s Alzheimer’s Disease Therapeutics Accelerator Projects, Penn Medicine researchers Ian A. Blair, George Kannarkat, David J. Irwin, Katheryn A.Q. Cousins are studying a novel approach to the treatment of Alzheimer’s disease.
Current Alzheimer’s drugs can slow memory loss but don’t fully stop the disease. This newly-funded project is exploring a new treatment approach by focusing on a protein called HMGB2, which may play a key role in brain cell damage.
The team discovered that in Alzheimer’s disease, HMGB2 levels are much higher and can trigger immune cells in the brain to mistakenly destroy healthy neurons. They plan to test drugs like selinexor and others that block HMGB2 to see if they can prevent this harmful chain reaction with the goal of developing new treatments that could better slow or stop the disease in the future.
“We anticipate that the proposed research will result in the discovery a therapeutic agent that (like selinexor) prevents HMGB2-mediated translocation of CRT from the ER to the plasma membrane of CNS cells,” explained Ian A. Blair, PhD, A.N. Richards Professor of Pharmacologyat the Perelman School of Medicine. “The therapeutic agent would inhibit microglial-induced phagocytosis, prevent neuron loss, and so provide a novel approach to stop the progression of AD.”
Farraday Johnson: From STAR Intern to Penn Memory Center Coordinator
 Last summer, Farraday Johnson joined the Penn Memory Center (PMC) as part of the Summer Training in Aging Research (STAR) program. Under the mentorship of Katheryn Cousins, PhD, she explored how biological age relates to plasma biomarkers linked to Alzheimer’s disease. During her internship, Johnson combined research, lectures, and clinical shadowing, gaining hands-on experience in aging and memory research.
Last summer, Farraday Johnson joined the Penn Memory Center (PMC) as part of the Summer Training in Aging Research (STAR) program. Under the mentorship of Katheryn Cousins, PhD, she explored how biological age relates to plasma biomarkers linked to Alzheimer’s disease. During her internship, Johnson combined research, lectures, and clinical shadowing, gaining hands-on experience in aging and memory research.
“One thing I will say about the STAR program is that you’re in very capable hands of professionals who will be able to guide you through every step,” Johnson said. “It is an amazing opportunity to be around such wise and experienced individuals.”
Building on her strong performance as a STAR intern, Johnson officially joined PMC in February as a clinical research coordinator for the Anti-amyloid Therapy Monitoring (ATM) study. In this role, she has learned to process blood samples, recruit participants, and work closely with clinicians—all while connecting with patients.
“Penn is my dream,” Johnson said, as she looks ahead to applying to medical school and continuing her work in Alzheimer’s research.
Source: Meghan McCarthy, Penn Memory Center, “Farraday Johnson’s Path from STAR Intern to PMC Coordinator,” Penn Memory Center, April 23, 2025
RESEARCH HIGHLIGHT: Clinical criteria for limbic-predominant age-related TDP-43 encephalopathy
In a recent publication authored by IOA Co-director David A. Wolk, MD, he and his team outline the first clinical diagnostic criteria for limbic-predominant age-related TDP-43 encephalopathy (LATE), a common but under-recognized cause of memory loss in older adults. Often mistaken for Alzheimer’s disease, LATE is linked to abnormal TDP-43 protein accumulation rather than amyloid pathology. The proposed criteria help distinguish LATE from Alzheimer’s by identifying specific patterns of memory decline, hippocampal shrinkage, and amyloid biomarker status. This important step forward lays the foundation for improving diagnosis, refining clinical trials, and developing targeted treatments for age-related memory disorders.
Read the full publication here.
Source: Wolk DA, Nelson PT, Apostolova L, et al. Clinical criteria for limbic-predominant age-related TDP-43 encephalopathy. Alzheimer’s Dement. 2025; 21:e14202. https://doi.org/10.1002/alz.14202

